Design & Control of Pharma Water System to Minimize Microbiological Contamination

Water-borne microorganisms are ubiquitous and varied in their ability to survive and grow under different conditions. Therefore, an out-of-control water system can cause harm to the patient or adulterate pharmaceutical products. Purification of water is required to prevent interaction with drug substances or other ingredients in the product formulation. Water must also be microbiologically controlled and monitored.
Both chemical and physical water-purification methods are based on robust technology that can, in most cases, reduce the level of contaminants to fewer than one part per million (or 1:10–6 ). These methods have an assay sensitivity in the range of parts per billion (1:10–9 ).
While chemical analysis usually generates answers quickly enough to ensure a rapid response to problems, microbiological assessment is often slower and less accurate. While rapid microbiological methods are gradually being implemented (such as ATP bioluminescence* or fluorescent DNA-specific dyes), most microbiological assessments of pharmaceutical-grade water rely on cultural methods.1 This means bioburden results are not available until several days have elapsed,2 placing considerable emphasis upon good design principles.
This article assesses some of the requirements for good design, together with the control measures necessary to maintain effective microbiological control in pharmaceutical facility water systems.
PHARMACEUTICAL-GRADE WATER
There are four grades of water in pharmaceutical production defined in the United States Pharmacopeia (USP) and/or European Pharmacopoeia (Ph. Eur.): potable (mains) water (USP), purified water (USP and Ph. Eur.), highly purified water (Ph. Eur.), and WFI, or water for injection (USP and Ph. Eur.). Potable water is the starting water for the other grades; it has no direct product contact. Each grade has microbial issues related to the method of production, degree of purification required, and process of storage and distribution.
Purified water, typically produced by reverse osmosis, is intended for use in formulations that are not intended to be sterile or apyrogenic (i.e., do not require an endotoxin specification). Uses include some oral and topical products, as well as the granulation processes for tablets and capsules. With such medications, the concern is with overall bioburden and the absence of “objectionable” microorganisms: those that pose potential patient harm, based on the route of administration.3 Purified water is also the feed for WFI and for pharmaceutical-grade clean steam. Highly purified water is intended for the preparation of ophthalmic, nasal/ear, cutaneous, and other medications, as required in the Ph. Eur. This grade of water requires both endotoxin (< 0.25 endotoxin units [EU] per milliliter [mL]) and bioburden control (< 10 colony forming units [CFU] per 100 mL).
WFI is the highest quality water used by the pharmaceutical industry; it is produced either by reverse osmosis or by distillation (according to both USP and Ph. Eur. since 2015). Bioburden and endotoxin control requirements are set out in the Ph. Eur. as < 10 CFU/100 mL (bioburden) and < 0.25 EU/mL (endotoxin). WFI is used for the preparation of parenteral medicines, dialysis, and irrigation solutions, as well as cleaning reagents in the highest-grade cleanrooms.
MICROBIOLOGICAL CONCERNS
Potable water from private water companies or municipalities is monitored to ensure that levels of chemical pollutants remain within established safety criteria, and screened for microorganisms including Escherichia coli, enterococci, Pseudomonas aeruginosa, and fecal coliforms.4 In most locales the quality of the water supplied to the pharmaceutical facility is satisfactory. As a safeguard, however, many facilities elect to test the water for organisms like E. coli as a marker for fecal contamination. Onsite potable water is treated, softened, purified (according to the grade required), and distributed.
While most well-designed water systems can be maintained in a state of control, microbiological problems can develop. Microbial adherence is a consequence of the balance of attractive and repulsive physicochemical interactions between bacteria the surface. The primary issue is biofilm formation—slime-like microbiological communities that occur when microorganisms adhere to a surface (such as pipework with a poor flow rate).
A biofilm develops because bacterial cells, once attached, secrete a polysaccharide known as glycocalyx (hydrated polymeric slimy matrices). The glycocalyx enables each bacterium to encapsulate itself
on the surface; biofilm forms as these organisms accumulate. The steps
involved in biofilm formation are (Figure 1):
- Individual cells populate the surface (initial attachment)
- Irreversible attachment
- Extrapolymeric substances are produced and attachment becomes
- irreversible
- Biofilm architecture develops and matures
- Single cells (or clumps of cells) are released from the biofilm over time
* Adenosine triphosphate (ATP) is a nucleotide found in all living organisms; ATP bioluminescence is used to monitor contamination.
- 6Jiminez, L. “Microorganisms in the Environment and Their Relevance to Pharmaceutical Processes.” In Microbial Contamination Control in the Pharmaceutical Industry, edited by L. Jiminez. New York: Taylor & Francis, 2004
- 9Sandle, T., and K. Skinner. “Examination of the Optimal Cultural Conditions for the Microbiological Analysis of a Cold Demineralized Water System in a Pharmaceutical Manufacturing Facility.” European Journal of Parenteral and Pharmaceutical Science, 10, no. 1 (2005): 9–14
- 1Cundell, A., O. Gordon, N. Haycocks, et al. “Novel Concept for Online Water Bioburden Analysis: Key Considerations, Applications, and Business Benefits for Microbiological Risk Reduction.” American Pharmaceutical Review, 16, no. 3 (2013): 26-31.
- 2Sandle, T. “Characterizing the Microbiota of a Pharmaceutical Water System-A Metadata Study.” SOJ Microbiology and Infectious Diseases 3, no. 2 (2015): 1–8.
- 3Sutton S. “What Is an Objectionable Organism?” American Pharmaceutical Review 15, no. 6 (2012): 36–48.
- 4Berry D., C. Xi, and L. Raskin. “Microbial Ecology of Drinking Water Distribution Systems.” Current Opinion in Biotechnology 17, no. 3 (June 2008): 297–302.
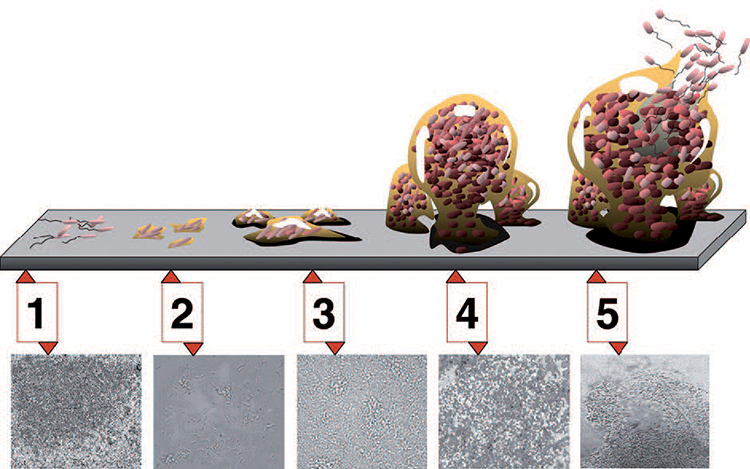
This type of attachment occurs relatively slowly. Various factors affect the process, including the type of bacterium involved, the size of the bacterial population in the environment, and the duration of its growth phase.5 In general, Gram-negative bacteria form biofilms more readily,6 due in part to appendages on the bacterial cell (fimbriae) that allow such them to attach to surfaces more easily. Surface charge is another important phenomenon in relation to bacterial adherence.7
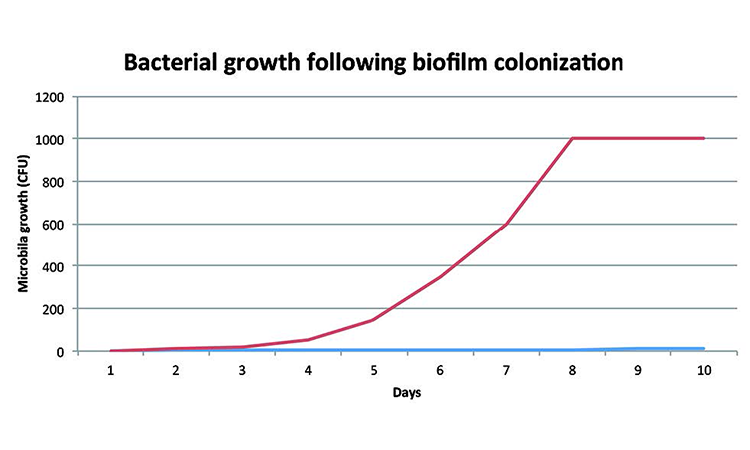
Microbial growth in a biofilm is often rapid at the point of source (Figure 2). The pattern from user outlines is often sporadic, however, because contamination is eluted from the biofilm at different rates over time.
- 5Sandle, T. “Bacterial Adhesion: An Introduction.” Journal of Validation Technology 19, no. 2 (2013): 1–10 http://www.ivtnetwork.com/article/bacterial-adhesion-introduction
- 6Jiminez, L. “Microorganisms in the Environment and Their Relevance to Pharmaceutical Processes.” In Microbial Contamination Control in the Pharmaceutical Industry, edited by L. Jiminez. New York: Taylor & Francis, 2004.
- 7Doyle, R. J. “Contribution of the Hydrophobic Effect to Microbial Infection.” Microbes and Infection 2, no. 4 (2000): 391–400.
Biofilms are of particular concern with water systems, since Gram-negative bacteria constitute the majority of the bacterial populations found in aquatic environments. These types of organisms, moreover, also shed endotoxins, a component of the cell wall.8
Many stages of the water-purification process can create conditions which, although designed to reduce contaminants, paradoxically promote biofilm formation. An example is in-depth filtration through a matrix. As water percolates through the filter, microorganisms are adsorbed onto the matrix, where they form complex communities. Further on, the purification pathway can create a series of colonizable environmental niches of varying nutrient richness. At the end of the process, which essentially depletes nutrients to very low levels, an extreme environment is created.9 This environment elicits extreme responses from any microorganisms present, making them difficult to eliminate.
For appropriately designed and maintained systems, purified water and WFI present low risks of microbial contamination. Whenever microorganisms are detected (and certainly when above specification), however, this creates a significant hazard.
GOOD DESIGN PRINCIPLES
While different phases of water generation can contribute to risks, there are a number of design and control steps that can reduce microbiological proliferation:10
Break tanks
Break tanks, which prevent water produced during production from reentering the water supply, are the first areas in which microbial colonization can occur. Microorganisms present in incoming potable water attach themselves to the sides and bottom of the tank, forming a biofilm. Samples taken from the tank usually meet the specification for potable water and give no immediate indication of the biomass that is accumulating. Regular maintenance and tank flushing are the main preventive measures.
Activated carbon beds
The bed matrix consists of finely divided charcoal, which is highly efficient at removing low-molecular-weight organic materials. It also oxidizes and removes additives such as chlorine. The vast surface area and accumulation of nutrients on the bed, combined with chlorine removal, can lead to rapid microorganism colonization and proliferation. Most of the organisms are Gram-negative bacteria and, should they undergo cell lysis, can be a source of endotoxins. An essential point of control over the entire water system is the ability to sanitize the beds regularly with hot water or steam, coupled with frequent replacement. Sanitization should begin at a higher frequency (such as weekly) for a new water system; this could be decreased over time (monthly) based on a microbial bioburden trend review. Six months to one year of data would be required to assess the bioburden pattern.
Water softeners
In areas with hard water, softeners are required to prevent interference with the deionizers and reverse osmosis systems. As water passes through the resin-filled columns, divalent calcium and magnesium cations are exchanged for sodium ions. The resin matrix provides an enormous surface area for potential microbial colonization, however. Sanitization and control measures such as ultraviolet light and chlorine are essential in maintaining water quality.
“Microorganisms present in incoming potable water attach themselves to the sides and bottom of the tank, forming a biofilm.”
Deionization devices
Standard deionization systems consist of charged resin columns. These may be separate for cation and anion removal, or may use a mixed-bed system. The advantage of deionization is that the columns require regeneration with 1 molarity (M) hydrochloric acid and 1M sodium hydroxide, both of which are strongly biocidal. If the regeneration frequency is high, the columns are maintained in a sanitized state. Unsanitized columns or those that are not regenerated for more than a couple of days present the same problems as activated charcoal beds, which is the risk of bacterial growth occurring.
Electrodeionization systems permit continuous column regeneration without the need to add regeneration agents. They are easy to maintain, but they also encourage bacterial growth. A reverse osmosis membrane will filter out bacteria, but growth can occur if not properly maintained. As fragments of the bacterial cell wall break off, endotoxins can easily pass through the membrane.
Storage and distribution systems
Poorly designed storage and distribution systems create opportunities for recolonization and, ultimately, product contamination. Colonization is often difficult to detect because biofilms release contamination slowly and randomly. (Microbial populations in water rarely indicate normal distribution, which means levels can appear and disappear over time before the overall trend can be discerned.)
Storage tanks
Water storage tanks are normally constructed from stainless steel. Where they are used, it is important to determine capacity, rate of use, and frequency of flushing and sanitizing the internal surfaces. Regular water turnover helps prevent contamination; slow turnover, on the other hand, presents a greater potential contamination risk. Storage tanks should be vented to manage water level fluctuations. To prevent microbial contamination from outside air, vents should be fitted with a hydrophobic air filter. Such filters are also used to avoid filter occlusion, which can create vacuum conditions and lead to tank implosion. Vent filter integrity testing should be performed regularly (e.g., once every 6 or 12 months).
Storage temperature
It is standard practice to store WFI in a recirculating stainless steel system, although on occasions polyvinylidene fluoride (PVDF) is used when very low mineral content water is needed. Recirculating systems that operate at temperatures of 65ºC to 80ºC are self-sanitizing, with the caveat that no cold spots below 65ºC form. Purified water systems can be hot or cold. Key aspects of cold water systems are discussed in more detail below.
Pipe and tank design
If they are poorly designed or improperly maintained, pipes and tanks are more likely than any other part of the water system to develop contamination. The general requirements for well-designed pipes are:
- Smooth internal surfaces. Microorganisms adhere less well to smooth surfaces than to rough surfaces, therefore corrosion resistance and avoiding rouging (iron oxide formation) is important (as can be achieved by the electropolishing of stainless steel). Pipe joints and welds can also disrupt smoothness.
- Continuous water movement in tanks and rapid flow in pipework; velocities in the range of 1–2 meters per second have been found to be satisfactory.11 This minimizes opportunities for microorganisms to adhere to surfaces (and form biofilms). Where shear forces occur, microorganisms adhere poorly to surfaces. Where there is no water movement, there is no shear (shear increases with the speed of flow).
- Avoid areas where water can remain stagnant:
- If a branch pipe is too long to allow the turbulence of the flowing main to disturb its contents, water may stagnate in “dead legs” (Figure 3). The principle is to always minimize the length of branch pipes.
- Water can also remain stagnant in valves, particularly at user points—and especially those that not in frequent and regular use. This can be counteracted by hygienic or “zero dead leg” valves which, although significantly better than the alternatives (say ball valves). This should not lead to a sense of false security, however, since they can harbor endotoxin-shedding biofilms. Having the correct sloping for drainage can also reduce contamination risk.
- Ring mains should be sloped (“drop”) from point of origin to the point of return to ensure that systems are completely drainable.
- Avoidance of leakage. Water leaks can cause bridging of water to the external environment through which bacteria may enter the system. Storage tanks should be equipped with filter on their air vents to prevent air-borne microbiological ingress. They may even be held under a “blanket” of an inert gas such as nitrogen.
- High temperature storage and distribution. The risks of endotoxin-shedding biofilms despite the best attempts at control above are thought to be so consequential that the most manufacturers require the temperature of storage and distribution to be maintained higher than 65°C. Lower temperatures may also be acceptable, provided the manufacturer has adequate data to demonstrate that a lower temperature works as intended.
- It should however be considered that 65°C is too high a temperature for most pharmaceutical formulation purposes. This means that user points are generally equipped with some form of cooling mechanism. It should be noted that heat exchangers used for this purpose may be a source of endotoxin and bacterial contamination and may thus cancel out many of the benefits of high temperature circulation.
- The use of coated surfaces on pipes and in tanks, where appropriate (as not to pose a risk of leaching toxic substances) can help to address bio-fouling.12
- 8Novitsky, T. J. “Bacterial Endotoxins (Pyrogens) in Purified Waters.” In Biological Fouling of Industrial Water Systems: A Problem Solving Approach, edited by M. W. Mittelman and G. G. Geesey. San Diego: Water Micro Associates, 1987.
- 9Sandle, T., and K. Skinner. “Examination of the Optimal Cultural Conditions for the Microbiological Analysis of a Cold Demineralized Water System in a Pharmaceutical Manufacturing Facility.” European Journal of Parenteral and Pharmaceutical Science, 10, no. 1 (2005): 9–14
- 10Sandle, T. “Avoiding Contamination of Water Systems.” Clinical Services Journal 12, no. 9 (2013): 33–36
- 11Noble, P. T. “Transport Considerations for Microbial Control in Piping.” Journal of Pharmaceutical Science and Technology 48, no. 2 (1994): 76–85.
- 12Thouvenin, M., V, Langlois, R. Briandet, et al. “Study of Erodable Paint Properties Involved in Antifouling Activity.” Biofouling 19, no. 3 (2003): 177–186.

Cold water systems
Systems for purified water typically use ozone, ultraviolet light, and in-line filters to maintain microbial quality instead of high temperature. Important points to consider are:
- Ozone is used periodically for sanitization. It attacks the outer surfaces of microorganisms and destroys cell walls and membranes.
- Ultraviolet light is not a sterilant, although it has some microbial reduction properties.13 Efficiency depends on path length, speed of flow, and age of the light source. The most commonly used wavelength for microbial reduction in pharmaceutical water treatment systems is 254 nanometers (nm). Ultraviolet light is also very useful for catalyzing the breakdown of ozone or hydrogen peroxide used as sanitizing agents, although its efficacy is often diminished by poorly maintained or malfunctioning lamps.
- Filters are ideal matrices for colonization; they need careful monitoring of pressure differentials and frequent sanitization or changing. If a biofilm has formed on a filter, sanitization will kill most microorganisms within the biofilm but will probably not remove the matrix, which may be rapidly recolonized. In addition, the presence of highly resistant “persister cells” within the population will remain unaffected and regrow.
- Cold water systems generally use thermoplastic materials because they suffer less biofouling than stainless steel (at low temperatures). Plastic material used to construct pipework is typically polypropylene or PVDF.
- Bends in pipework should be as gentle and as few as possible; tap points should be kept to a minimum. Any disruption to the smooth flow of water results in turbulence, which assists biofilm formation by creating more opportunities for circulating microorganisms to adhere to colonizable surfaces.
User points
Water points in production areas involve the transfer of water from the circulating water loop to the point of use via transfer piping (or tubing), which should be made of a suitable nontoxic material, such as polyvinyl chloride (PVC), chlorinated PVC, polypropylene, or PVDF. The transfer piping should be drained after use and changed regularly (such as every 24 hours); care must be taken to avoid splash-back from sinks or recontamination from aerosols. New tubing should be sanitized before fitting; it is also common for the tubing and outlet to be flushed prior to use (for a defined time or given volume of water). These measures are taken to avoid contamination of the water during the transfer process.
WHEN THINGS GO WRONG
Loss of water system control and microbial contamination can have a number of causes, including aging resin, aging filters, poorly maintained ultraviolet lights, improper maintenance, failure to achieve effective heat distribution, leaks (such as heat exchangers), dead legs, and water-system modifications (e.g., cutting through pipework).
Treating water systems
Several options are available for treating and improving water quality. The method chosen depends on what is causing the microbial deterioration, the source of the problem, the water quality required, the volume to be treated, and the type of distribution system. System design can influence the size of the microbial population and the ability to remove it. Dead legs, long pipework runs to taps, undrainable pipes, and U-bends can also create microbiological problems.
Four methods are routinely used to remove microbial contamination: heat, chemicals, filtration, or ultraviolet light. Contact time is important for each one.
Heat, as described earlier, can be used in the circulating loop of a hot-water system maintained at 65°C to 80°C (with ≥ 75°C being optimal). If this proves insufficient there should be the capacity to superheat the system (taking the temperature up to 121°C for one hour or longer).
Chemical treatment (e.g., ozone, chlorine, chlorine dioxide, hydrogen peroxide, peracetic acid, and sodium hydroxide) is applicable to raw mains water, but can also be used to treat distribution systems of water produced by distillation, deionization, and reverse osmosis. Concentration of the chemical used depends on the location of the water in the distribution system. Chlorination, for example, is generally effective if minimum levels of 0.2milligrams (mg) per liter (L) of free chlorine are attained. The contact time will vary with water temperature and pH; typical times for 0.2mg/L of free chlorine are between 30 and 60 minutes. Importantly, any chemical added must, at some point, be removed.
Membrane filtration using a 0.22 micrometer (μm) porosity filter is applicable where usage is moderate and continuous water circulation can be maintained (i.e., water is continually returned to the storage tank and refiltered, except what is drawn off for use). While filtration works well in principle, it is relatively expensive for high throughputs because they need regular changing to prevent blockage and “grow-through.” For this reason, using 0.22 μm filters to control contamination in water used for product manufacture is frowned upon. Filters should be used only prior to the distribution process.
- 13Powitz, R. W., and J. Hunter. “Design and Performance of Single-Lamp, High-Flow Ultraviolet Disinfectors.” Ultrapure Water 2

“Most of the organisms are gram-negative bacteria, and should they undergo cell lysis, can be a source of endotoxins”
Ultraviolet radiation (254 nm) is used to disinfect water of good optical clarity; it works particularly well in a recirculating system where water flows over a multiple lamp system. While contact times vary according to dose and flow rate, they are normally in the region of 1 to 10 seconds. This time is required to allow UV light to penetrate through the water and make contact with any bacteria present.
MICROBIOLOGICAL MONITORING
Frequent monitoring is important to verify microbiological control. This involves a bioburden assessment, typically using microbial count methods with membrane filtration, and a low-nutrient agar such as R2A as the method of choice (Figure 4). Where applicable, a Limulus amebocyte lysate test for bacterial endotoxin is also recommended. In both cases, action or alert limits must be based on validation data and must be set low enough to signal significant changes from normal operating conditions.
CONCLUSION
In pharmaceutical water-distribution systems, microbial adhesion will initiate biofilm formation, exacerbating contamination of water, reducing the aesthetic quality of potable water, increasing the corrosion rate of pipes, and reducing microbiological safety through increased survival of pathogens. Microbial control, therefore, is a matter of concern for engineers, production personnel, and microbiologists.
This article has outlined the microbiology of water systems and provided an overview of the design, control, and generation of pharmaceutical-grade water. While several aspects of design and control have been discussed, perhaps the two most important are to avoid standing water (which is invariably a source of contamination) and to have provisions for sanitization in place at each step of the water system.
Editor’s note: Items 1–5 in the “Pipe and tank design” section of this article have been reprinted from Pharmaceutical Microbiology: Essentials for Quality Assurance and Quality Control, by Tim Sandle, Chapter 10: Assessment of Pharmaceutical Water Systems,” page 120 (2015), with permission from Elsevier.


