The creation of a new ICH guidance document, Q13,1
- 1International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. “ICH Harmonised Tripartite Guideline Q13: Continuous Manufacturing of Drug Substances and Drug Products.” Published July 2021.
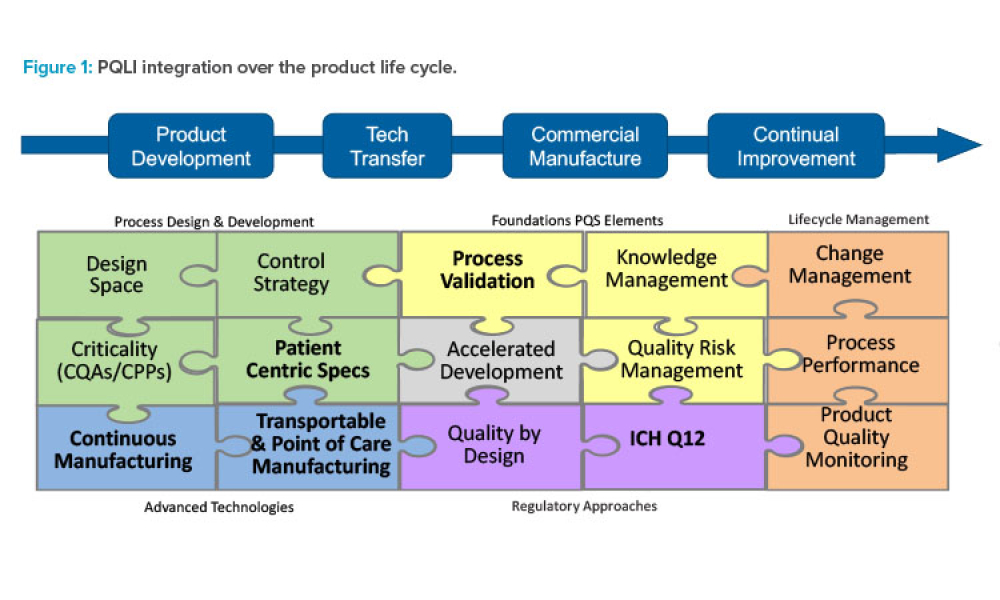
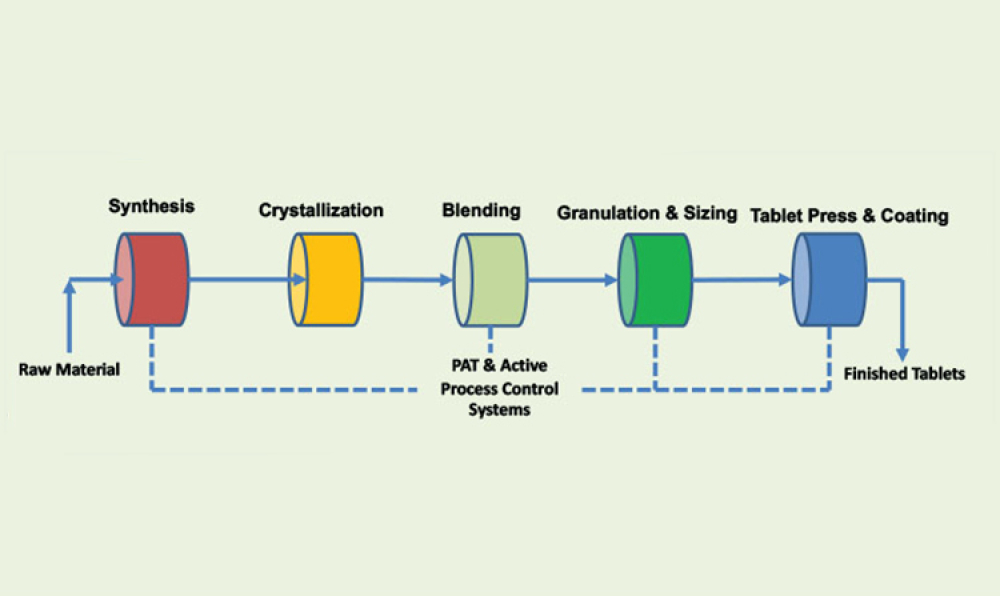
The creation of a new ICH guidance document, Q13, presents an opportunity for industry and regulators around the world to connect and develop harmonized regulatory expectations for the continuous manufacturing (CM) of drug substances and drug products, resulting in an increased likelihood of implementation across the globe.