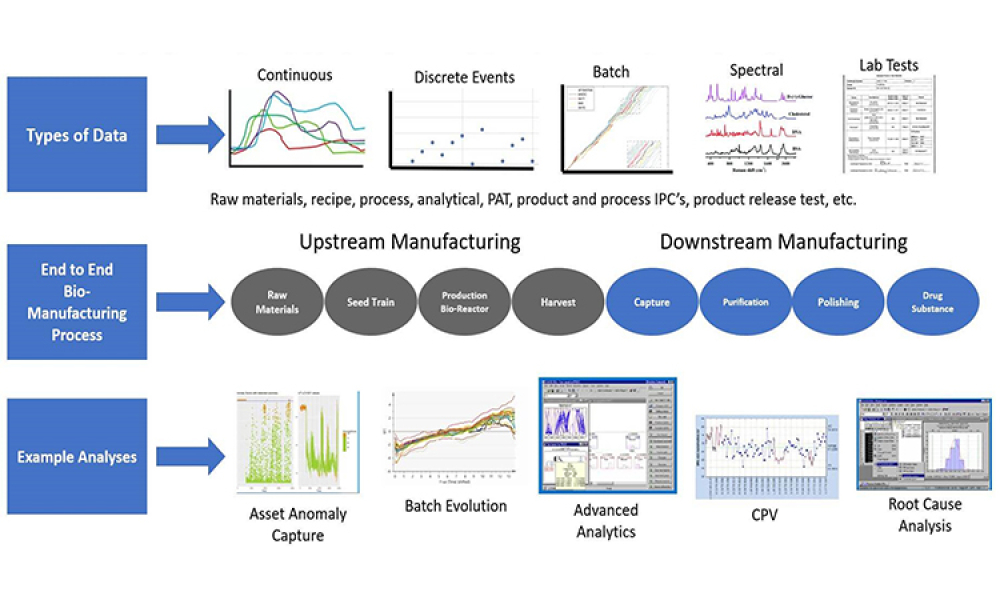
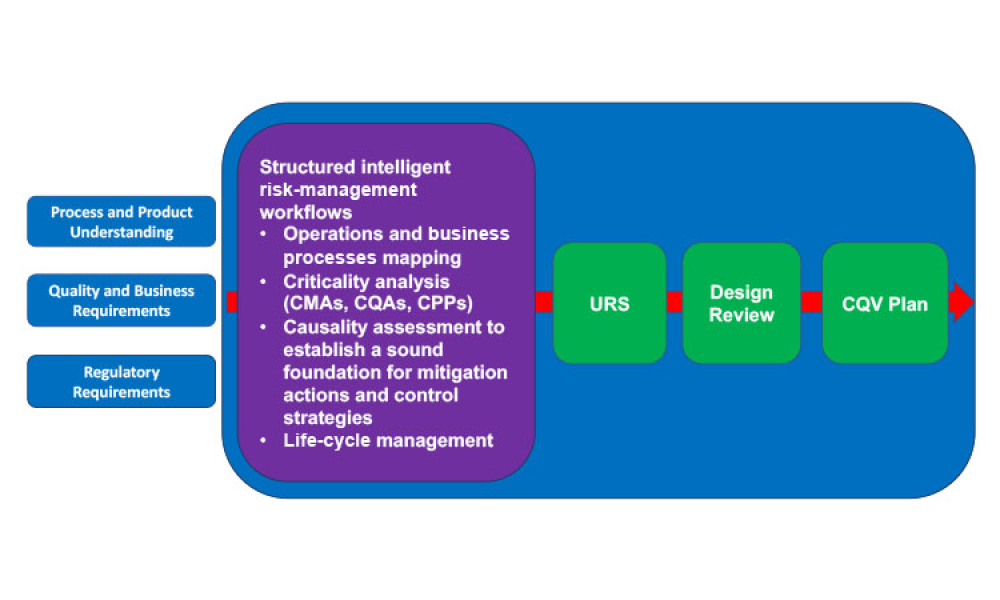
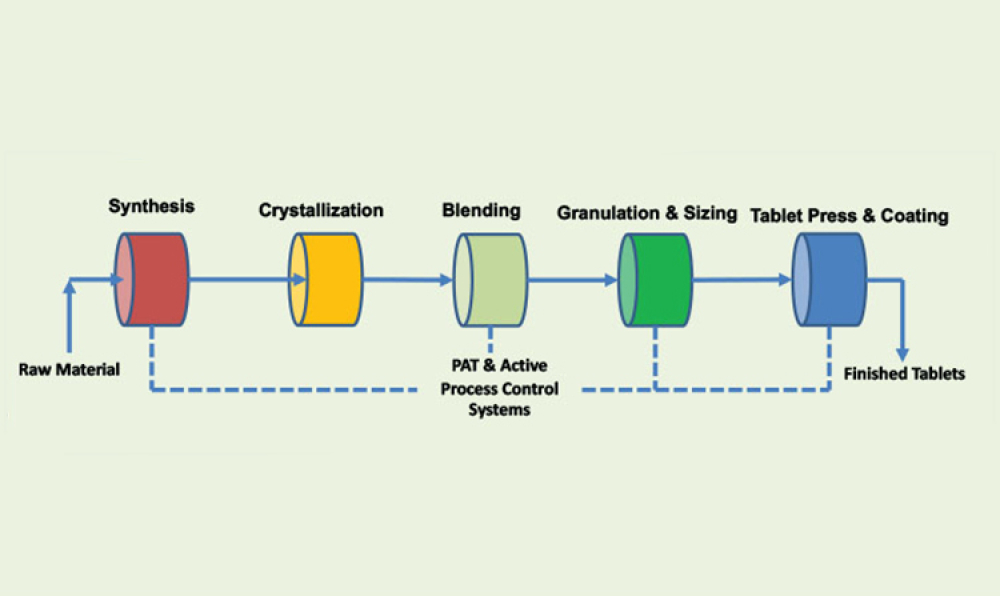
The 2022 Planning Committee is delighted to welcome attendance and registration for the fast-approaching ISPE Biotechnology Conference, taking place 28-30 June in Boston, Massachusetts and virtually.
Robert Dream, PE, CPIP, PhD

HDR COMPANY LLC
Managing Director
Robert Dream is an industry leader with more than 29 years’ experience, including 15 years of executive leadership experience, in the consumer product, pharmaceutical, biotechnology, and life sciences industries. He has led projects, improved processes, and scaled up products through operational excellence strategies and technology knowledge and know how. He is business oriented and has a functional knowledge of manufacturing, operations, regulatory compliance and regulatory affairs (assisting on CMCs and BLAs), risk mitigation and risk management. He is experienced in biotechnological and biological products manufacturing (including Continuous Biomanufacturing, ATMPs/Cell & Gene Therapies), with extensive hands-on, senior managerial and executive experience at world-leading organizations. He is a registered professional engineer and an active member of ISPE and PDA. He is a member of the Pharmaceutical Processing Editorial Advisory Board, the Pharmaceutical Manufacturing Editorial Advisory Board, Pharmaceutical Technology Editorial advisory Board, Pharmaceutical Engineering Committee, and the INTERPHEX Advisory Council. He is a graduate of the Illinois Institute of Technology (BS and MS) and Drexel University (PhD).